Scientifics
Snowflakes
Rosanna Gullveig

As I write this article, a flurry of little white specks are falling from the sky. It seems that winter has finally arrived in my little area of the world! As I watch the snow gently cover every surface in sight, I can’t help but wonder... do all snowflakes look alike? And how do they get those fanciful shapes?
So this month, I’ve decided to tackle those very questions.
What ARE snowflakes?
First things first, what exactly are snowflakes? Snowflakes are a form of frozen water. When water freezes, the water molecules form crystals of ice. The word “snowflake” is actually a general term than can either mean a single ice crystal or a whole bunch of them stuck together (which are those puff balls we all <3 so much!).
Now, how do the water molecules make those pretty shapes? The answer to that lies in the chemistry of water. This is a scientific article, after all, you didn’t really think I wouldn’t tackle that, did you? *EG*
A water molecule is composed of two hydrogen atoms (the white spheres in the diagram below) and one oxygen atom (the red sphere). When water freezes, the molecules make bonds between them (these are called Hydrogen Bonds). These bonds make the molecules arrange themselves in an ordered and symmetrical way, thus maximizing attractive forces and minimizing repulsive forces. Simply put, this means that the water molecules make a specific pattern, which forms an ice crystal.
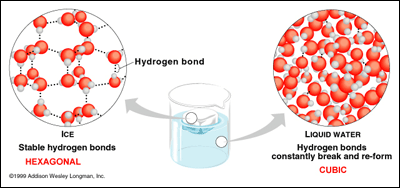
What about snowflake shape?
The actual shape of a snowflake depends on the environmental conditions during which it was formed. Factors such as temperature, wind, humidity and dirt all play a role in the snowflake’s eventual shape.
A snow crystal forms when water vapour condenses directly into ice. Depending on the temperature at which this happens, the shape of the crystal varies. These shapes include dendrites, columns, rods, and stars, among others. Most snowflakes have a hexagonal shape (that means 6-sided). This is due to the six-fold symmetry of the ice lattice, which is the crystal structure the ice makes. This basically means that the shape we commonly associate with snowflakes is due to those bonds between the water molecules I talked about earlier.
As the ice crystal is carried in the clouds, it continues to grow as more and more water molecules get attached to the basic structure. Once again, depending on the temperature, the new crystals could have a different shape than the previous crystals, which is why snowflake structures are often complex and varied. This also means that the exact structure of the snowflake depends on the temperature, but other factors can also play a large role:
o Humidity, which determines how much water is in the air (water saturation), influences how many more water molecules are added to the structure.
o Specks of dirt can deform the shape and create uneven sides to the flake.
o Wind can make the snowflake go up and down within the clouds, giving it more opportunity to grow into a complex shape due to the common temperature fluctuations.
The following diagram illustrates the different shapes an ice crystal will form, depending on the temperature. Most snowflakes are amalgams of a number of these shapes, all at once.
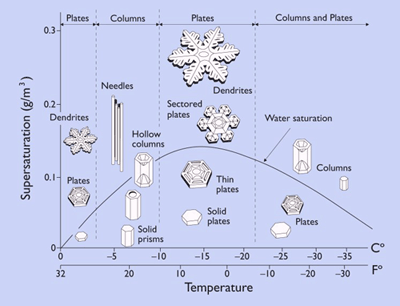
Whoa!! Who knew making snowflakes was such a complicated process? Did you know there are people who research snowflakes for a living? Yup, there are even people who do graduate studies in the field. Can you imagine that? “Hi, my name is blablabla and I have a PhD in snow”! Now, for the real question...
Is it true that no two snowflakes are identical?
Yes and no. Because there are so many variables involved in the process, it’s not likely that you’d find two identical snowflakes, and that’s not even counting some of the more complex issues such as electron spin, the exact number of molecules, isotope abundance, and the super-cooling of water (yeh.. watch me just name those concepts instead of actually trying to explain them, ‘cause some of them are the subject of entire university courses!). This means that the probability that you would find two identical snowflakes at any one time is REALLY small. Now add the fact that a snowflake’s shape in constantly changing as it falls towards the ground, and that most snowflakes are not perfectly symmetrical due to deformities and imperfections, and the probability becomes even smaller!
However, this being said, it is POSSIBLE that a very simple snowflake could have a shape-twin. Simple snowflakes such as needles and columns often have simple cylindrical shapes, for which there are fewer possible permutations. Furthermore, it is also POSSIBLE that a complex snowflake has had a twin at some point in history because, let’s face it, it’s been snowing for many millennia and simple statistics suggest that this would be possible.
So there you have it! The next time you complain about having to shovel the walk or about school being cancelled due to a snow-day (oh, wait... who’d be crazy enough to complain about that?), take a moment to marvel at the beauty and intricacy that is a snowflake. Who knows, maybe you’ll be lucky enough to find two identical ones!
References:
A Snow Primer: http://www.its.caltech.edu/~atomic/snowcrystals/primer/primer.htm
Snowflake chemistry: http://chemistry.about.com/od/moleculescompounds/a/snowflake.htm
Ice structure: http://fig.cox.miami.edu/~cmallery/150/chemistry/ice.jpg
Snowflake morphology: http://www.its.caltech.edu/~atomic/snowcrystals/primer/primer.htm